4-Methyl-1,2,3-Triazoles as N -Acetyl-Lysine Mimics Afford Potent BET Bromodomain Inhibitors with Improved Selectivity.
Cui, H., Carlson, A.S., Schleiff, M.A., Divakaran, A., Johnson, J.A., Buchholz, C.R., Zahid, H., Vail, N.R., Shi, K., Aihara, H., Harki, D.A., Miller, G.P., Topczewski, J.J., Pomerantz, W.C.K.(2021) J Med Chem 64: 10497-10511
- PubMed: 34236185
- DOI: https://doi.org/10.1021/acs.jmedchem.1c00933
- Primary Citation of Related Structures:
7MLQ, 7MLR, 7MLS - PubMed Abstract:
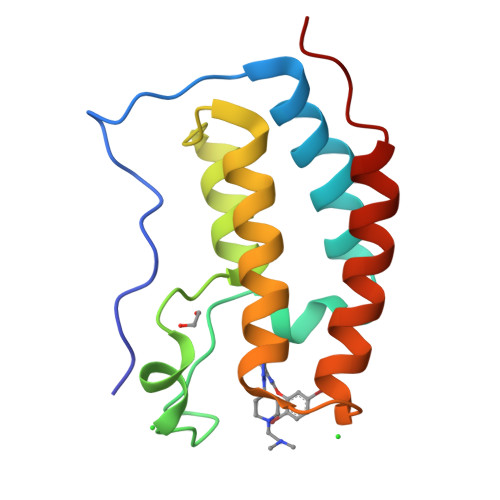
The bromodomain and extra terminal (BET) protein family recognizes acetylated lysines within histones and transcription factors using two N-terminal bromodomains, D1 and D2. The protein-protein interactions between BET bromodomains, acetylated histones, and transcription factors are therapeutic targets for BET-related diseases, including inflammatory disease and cancer. Prior work demonstrated that methylated-1,2,3-triazoles are suitable N -acetyl lysine mimetics for BET inhibition. Here we describe a structure-activity relationship study of triazole-based inhibitors that improve affinity, D1 selectivity, and microsomal stability. These outcomes were accomplished by targeting a nonconserved residue, Asp144 and a conserved residue, Met149, on BRD4 D1. The lead inhibitors DW34 and 26 have a BRD4 D1 K d of 12 and 6.4 nM, respectively. Cellular activity was demonstrated through suppression of c-Myc expression in MM.1S cells and downregulation of IL-8 in TNF-α-stimulated A549 cells. These data indicate that DW34 and 26 are new leads to investigate the anticancer and anti-inflammatory activity of BET proteins.
Organizational Affiliation:
Department of Chemistry, University of Minnesota, 207 Pleasant Street SE, Minneapolis, Minnesota 55455, United States.